

| Archive Blog Cast Forum RSS Books! Poll Results About Search Fan Art Podcast More Stuff Random |
|
Classic comic reruns every day
|
1 {photo of scientists at the National Ignition Facility (NIF) at Lawrence Livermore National Labs}
1 Caption: Working to Fusion
|
First (1) | Previous (3266) | Next (3268) || Latest Rerun (2886) |
Latest New (5380) First 5 | Previous 5 | Next 5 | Latest 5 Annotations theme: First | Previous | Next | Latest || First 5 | Previous 5 | Next 5 | Latest 5 This strip's permanent URL: http://www.irregularwebcomic.net/3267.html
Annotations off: turn on
Annotations on: turn off
|
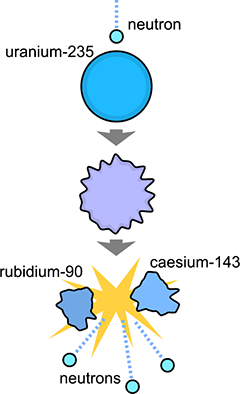 Nuclear fission of uranium-235 showing sample product isotopes. Image adapted from public domain image from Wikipedia Commons. |
There are other processes that can transform atoms by rearranging the numbers of protons and neutrons in the nuclei. A large atom can split apart into two smaller atoms, also ejecting some of the excess neutrons during the reaction. A few isotopes of heavy elements can undergo this process as a form of natural radioactive decay. Our old friends uranium-235 and uranium-238 both decay in this way a small fraction of the time (less than one in a million decays) rather than by alpha decay. The products can be various atoms such as caesium, zirconium, iodine, molybdenum, zinc, and so on.[1] This form of radioactive decay is called spontaneous fission.
Fission can also occur in another way. If a heavy nucleus is struck by a smaller particle, the collision can crack the nucleus apart. Neutrons are the best impacting particle, since they are very common, relatively heavy as sub-atomic particles go, and electrically neutral, which means they aren't repelled by the positively charged nucleus as an incoming proton would be.
When a neutron of roughly the right kinetic energy hits a nucleus of a heavy atom, such as uranium or plutonium, the nucleus splits into two smaller atoms. As mentioned above it also spits out some new neutrons, three in the case of the isotope uranium-235 (the impacting neutron is absorbed and three new neutrons are released, so there are two more going out than coming in). The interesting thing is that these neutrons generated by the fission reaction have the right energy to split apart other uranium-235 atoms if they happen to hit any. And if that happens, the three atoms split will emit three more neutrons each. They can split nine more uranium atoms, generating 27 more neutrons. And so on.
A small lump of uranium contains trillions upon trillions of atoms. A piece the size of a pea contains about 1020 atoms. If an energetic neutron hits that piece of uranium, it can start a chain reaction that very quickly splits apart every uranium-235 nucleus.
Now here's the thing. A uranium-235 nucleus might split, for example, into rubidium-90 plus caesium-143. But a uranium-235 nucleus contains more mass than a nucleus of rubidium-90 plus a nucleus of caesium-143 plus two extra neutrons. Even though the uranium-235 nucleus contains 92 protons and 143 neutrons, and the total numbers of protons and neutrons after the reaction are the same. The numbers of each particle are conserved, but the mass is not. What's going on here?
 Velcro works on short scales only, like the strong nuclear force. Creative Commons Attribution-NonCommercial image by Flickr user tanakawho. |
So each atomic nucleus (more complicated than that of hydrogen-1 with just a lone proton) actually contains less mass than the total mass of the individual protons and neutrons that make it up. What's more, the size of this difference, or mass defect, depends on the size of the nucleus in a slightly complicated way. Small nuclei experience a small amount of strong nuclear force, so they are held together relatively weakly. As the nuclei grow larger, the strong force holding the protons and neutrons together grows. This is similar to how small objects can be pulled apart fairly easily, but objects the size of Earth cannot because the gravitational force holding it together is so much bigger. So larger nuclei are held together more strongly, and the size of the mass defect per particle grows.
But then things get more complicated. The strong nuclear force is not like gravity or electromagnetism at all. Gravity has an unlimited range. It falls off moderately quickly with distance, but our sun still has a significant gravitational effect on Earth, the other planets, and even on nearby stars. The nuclear force is very strong at extreme close range, but drops to zero just a short distance further out. It's more like Velcro. Get two objects within a millimetre or so of each other, and Velcro will hold them together strongly. But at five millimetres range, Velcro is absolutely powerless. The strong nuclear force happens to fall off to zero once the nucleons (protons or neutrons) get about as far apart as the size of half a dozen nucleons. Now imagine the nucleus of a large atom. It's a ball made of protons and neutrons stuck together by the strong force. If that ball is big enough the nucleons on one side of it will be far enough away from the nucleons on the other side that they won't feel the strong force from them. So as the nuclei get bigger and bigger, the overall binding effect of the strong force, per nucleon, starts becoming weaker again.
There's a turnover point where the mass defect per nucleon is the greatest, and that happens to occur for the atom we know as nickel-62, with 28 protons and 34 neutrons. Nickel-62 is actually a fairly rare isotope of nickel, making up about 3.6% of naturally occurring nickel - the most common isotope is nickel-58. The mass-defect-per-nucleon curve is very flat around mass number 62, and two isotopes of iron also have mass defects per nucleon of almost the same amount as nickel-62. These are iron-58 and iron-56. Of these three isotopes, iron-56 is by far the most common, making up almost 92% of all the iron found in nature.[2]
 A nuclear explosion. Public domain image from Wikipedia Commons. |
So, if we take our lump of uranium-235 and fire a neutron at it, it splits an atom and releases three new neutrons plus quite a lot of energy. The new neutrons can split more atoms in the cascade described above, and each atom releases more energy. The result, if this reaction continues cascading uncontrollably like this, is a nuclear meltdown in which the lump of uranium rapidly melts - or, if it can't melt and flow away from itself fast enough, a nuclear explosion. This is the essential principle of a nuclear bomb. There are details in how you trigger the reaction and how you make the bomb safe before you trigger it, but they're not important right now (and would probably get me stuck on some sort of intelligence watch list if I talked any more about them).
On the other hand, if you can control the number of energetic neutrons somehow so they don't get out of hand, and siphon off the generated energy fast enough, you can produce a nuclear reaction that can serve as the basis for power generation. This can be achieved by putting the reacting material in close proximity to some material that absorbs neutrons. Graphite and water are good materials, and both are used in nuclear reactors of various designs. By moving these materials suitably, you can control the number of neutrons and turn the reaction up or down or off altogether. And this is basically how nuclear power plants work. A good nuclear power plant design will have enormous numbers of safety interlocks and failsafes to ensure the reaction can't get out of control, but there can still be unforeseen risks, alas.
 We need helium for stuff like this. Creative Commons Attribution-NonCommercial image by Flickr user one.juniper. |
The biggest bang for your buck from fusion comes from fusing together the very lightest atoms of all, hydrogen. If you can manage to get two protons (hydrogen nuclei) and two neutrons together, they will fuse into a helium nucleus. The helium nucleus has a significantly lower mass than the four individual particles, and the remainder is converted into energy and released. The trouble is it's extremely difficult to get four particles like that close enough together all at once for the strong nuclear force to start working on them.
An easier road comes if you take two atoms of the isotope hydrogen-2, each of which has a nucleus consisting of one proton and one neutron. Hydrogen-2 is also known by the name deuterium, and it occurs naturally on Earth at a rate of about 0.016% of all hydrogen atoms. If you mash just two of these nuclei together, they can fuse into a helium nucleus, releasing energy as they do so. The most ready source of deuterium we have is actually in plain old water. 0.016% of the water molecules contain an atom of deuterium instead of regular hydrogen. Such water molecules are called heavy water and can be separated and concentrated by a fairly simple process.
So theoretically we can release huge amounts of energy from water by fusing the deuterium into helium. There are also other possible fusion reactions, for example involving hydrogen-3, or tritium. The good thing about nuclear fusion is that it poses none of the risks of nuclear fission. We don't have to mine expensive uranium ores from the ground. We don't have to be careful about controlling the reaction lest it turn into a bomb, since it doesn't work on the same neutron chain reaction as fission - if something goes wrong, the reaction just stops. And it doesn't produce dangerously radioactive waste products like rubidium-90 and caesium-143 and other scary isotopes (it actually makes helium, which is a scarce natural resource that we could use more of!).[3] The main problem with nuclear fusion is we don't have the technology to do it yet, at least in a way that generates a reaction that produces more energy than we have to put in to get the fusion started.
That lies in the future, but it's a future worth pursuing. Nuclear fusion, if we can get it to work here on Earth, could potentially solve the major problems we face with our energy supplies at the moment - mostly the fact that most of the energy we use currently comes from sources that are dangerous to the short and/or long term health of our own civilisation.
[1] Any particular fission reaction can produce a range of possible product isotopes. For a full list, and the various probabilities of producing each of the different product isotopes, see this Wikipedia page on fission product yield.
[2] And we will meet iron-56 again later on. It's a very important isotope in the cosmic scheme of things.
[3] Some fusion reactions produce very high energy neutrons, which can hit material around the reaction and convert atoms into radioactive isotopes. So there is potential there to produce dangerous waste products, but this can be avoided with judicious reactor design.
|
LEGO® is a registered trademark of the LEGO Group of companies,
which does not sponsor, authorise, or endorse this site. This material is presented in accordance with the LEGO® Fair Play Guidelines. |