

| Archive Blog Cast Forum RSS Books! Poll Results About Search Fan Art Podcast More Stuff Random |
|
Classic comic reruns every day
|
1 {photo of a juggler playing with fire, which is blurred out by the rapid motions}
1 Caption: Motion and Heat
|
First (1) | Previous (3201) | Next (3203) || Latest Rerun (2895) |
Latest New (5380) First 5 | Previous 5 | Next 5 | Latest 5 Annotations theme: First | Previous | Next | Latest || First 5 | Previous 5 | Next 5 | Latest 5 This strip's permanent URL: http://www.irregularwebcomic.net/3202.html
Annotations off: turn on
Annotations on: turn off
|
So we've set the stage by talking about matter. Now it's time to make it come to life by providing the stage directions.
Matter is stuff. It is anything you can touch (at least conceptually, if you could get there). You are made of matter, your dog is made of matter, that tree outside is made of matter, your home is made of matter, your pizza is made of matter, the air you breathe is made of matter, planets and stars are made of matter. Everything you see around you is made of matter[1]. That's a lot of stuff.
 Things moving around. |
Enter: energy.
Energy is a more difficult thing to define, because it's not so immediately tangible as matter. Energy is not stuff. Energy is the ability to do stuff. If matter is the nouns of the universe, energy is kind of like the verbs, the "doing words" as generations of school teachers have explained to generations of kids. To make a ball, you need matter. To throw a ball, you need energy[2].
Things moving around is one of the most obvious manifestations of energy. A scene in which everything is sitting still has less energy in it than a scene containing the same objects moving around. The faster the objects are moving, the more energy they have. In fact, there's a quantitative definition of energy, which tells us exactly how the speed of an object is related to the energy it has.
The energy of a moving object depends on not just the speed it is moving, but on the square of that speed (i.e. the speed multiplied by itself). This means that if an object is moving twice as fast, it doesn't have twice the energy, it has 2×2, or four times as much energy. An object moving three times as fast has nine times the energy, and so on. This is why car crashes rapidly get much more dangerous as you go up in speed. The amount of energy that the car's body (and the passengers) need to absorb in a crash increases four times for every doubling of speed.
 A really massive moving object. |
The upshot of the previous two paragraphs is that speed is considerably more important than mass when thinking about energy. This is why bullets are so dangerous, because they move so fast. Make a bullet 50 times heavier, but slow it down by 50 times, and you have approximately the mass and speed of a cat running into you. You'll notice it, but it's not going to rip a hole through your body. Luckily for those of you who own cats.
The next thing to think about is: Where do moving objects get their energy? Let's think about a car. A car accelerates using a motor, which is a device for generating motion[3]. But it can't just produce that energy of motion out of nowhere, it has to convert the energy from some other form. Energy of motion, which we call kinetic energy, is only one type of energy. Another type of energy is the energy which is locked up inside chemicals such as petrol (or gasoline for the Americans), and which can be released by burning or other chemical reactions. This sort of energy just sits there for the most part, without any obvious signs of its presence, apart from the fact that the chemical has the potential to release its stored energy. So this sort of energy is called chemical potential energy.
 Sources of chemical potential energy. |
Heat, let's talk a little bit more about now. There's lots to say about heat, so we're not going to cover it all today. You probably have a reasonable idea of what heat is from your everyday experiences. Some things feel hotter than others, and when you put hot and cold things together the cold thing gets warmer and the hot thing gets cooler.
It seems like heat might be some sort of substance that flows from hot things into cold things. And for a long time that's exactly what people thought it was. Initially heat as an entity in itself was confused with the process of burning (for fairly obvious reasons). It took the 18th century French "father of chemistry", Antoine Lavoisier, to realise that burning wasn't releasing a substance with mass (phlogiston, more of which another day); in fact when objects burnt they became heavier. He recognised that the reaction of burning was a chemical combination of the burning object with oxygen from the atmosphere. But this left the question of where the heat came from. Lavoisier came up with the idea that heat was some other sort of substance, a "subtle fluid", which he named caloric. Demonstrably, caloric could have no mass, but it seemed possible that it was a massless physical substance that carried the property of heat.
But try as they might, nobody could extract and isolate caloric and put it in a jar by itself. Nevertheless, the caloric theory allowed the investigation of other aspects of heat and chemistry. It in fact turned out to be a very valuable way of thinking about heat, at least until Benjamin Thompson, the Count Rumford and his experiments in 1798. Observing the boring of a cannon in Munich, Rumford noted that the cannon and the drill heated up. He arranged an experiment using a blunted drill to see how much heat the cannon could be made to produce. His experiment showed that the cannon seemed to have an inexhaustible supply of caloric. As long as you kept drilling it, more heat would come out. He concluded it must be the action of the drilling itself that produces the heat, rather than it merely being something that releases heat from within the cannon. This is an important distinction, because it implies that the total amount of heat is not conserved, as it should be if heat is a physical substance. Heat can be produced from thin air, it seemed, by doing nothing other than rubbing materials together.
 Cannon overlooking Fremantle Harbour. |
In the mid-1840s, James Prescott Joule performed an experiment in which he used a stirring device to introduce a very carefully measured amount of kinetic energy into a container of water. He observed that the water heated up, and measured the rise in temperature. He discovered that the more kinetic energy he put into the water by stirring it, the warmer the water got, in precise proportion. He was able to measure how much kinetic energy would raise the temperature of the water by a certain number of degrees Fahrenheit.
This amount depended on the mass of the water. To heat a pound of water by 1 degree Fahrenheit required a certain amount of kinetic energy. To heat it by 2 degrees required precisely twice as much energy. And to heat two pounds of water by 1 degree also required exactly twice as much energy as heating one pound by the same amount. James Joule had discovered that the amount of heat in a substance was directly related to how much energy had been put into that substance. In other words, heat was equivalent to energy—heat was a form of energy.
But how does this work? Where does the kinetic energy go when things are heated up in this way?
Here's where our new friends, atoms, come in. What do atoms have to do with heat? I'm glad you asked!
There's a simple experiment you can do with a bicycle pump. Place your thumb over the hole where the air comes out and pump the handle. By blocking the hole with your thumb, you're not allowing the air to escape when the back end of the pump pushes on it. The result is that the air gets compressed inside the pump barrel. You'll also notice that the air pressing on your thumb (and possibly the whole pump) gets warmer. The air inside is heating up, because you are applying kinetic energy to it by pushing the pump handle in.
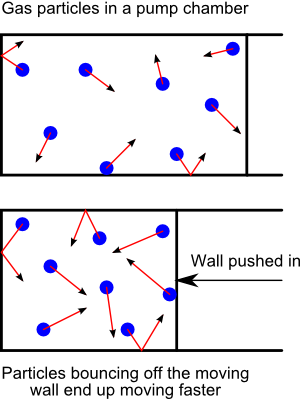 |
Now wait just a second. This means that the process of compressing a gas by pushing a wall into it is making the particles in the gas move faster. In other words, it's adding kinetic energy to the particles in the gas. And we end up feeling this as heat. Heat in a gas is actually the kinetic energy—the energy of motion—of the atoms in the gas! And this applies to all materials. Heat is just the atoms within the material moving faster. And if you do the calculations, you can figure out just how fast the atoms in a substance must be moving in order to have the amount of heat that they do.
So heat is a form of energy, but it's actually a form of kinetic energy, the energy of motion! This is a very amazing thing. The existence of atoms allows us to understand what heat actually is, and how it can be transferred to substances by putting kinetic energy into them. And when you put a hot thing next to a cold thing, the kinetic energy of the atoms in the hot thing transfers, through tiny collisions between the atoms, from the hot thing to the colder thing. In other words, the heat energy flows from the hot thing to the cold thing. And all without needing any pseudo-mystical substance like caloric to explain it.
There are other forms of energy than the ones I've discussed today. One in particular is very important, and if you're wondering when I'd mention it... it'll be in another annotation. For now, let's just end with the fact that energy, despite not being made of matter, is something that has very specific and measurable effects on matter. We can measure energy reliably, and we know that energy is neither created nor destroyed. It can be converted between different forms, and those conversions are responsible for all of the amazing things that we, and the universe around us, does.
And if we can measure energy, we should give it a unit. In the same way that we measure distance in metres (or feet, or whatever), and we measure mass in kilograms (or pounds, again, depending what particular units you want to use), we can measure energy. In the International System of Units based on the metric system, the basic unit of energy is, very appropriately, named the joule, after James Prescott Joule.
[2] This analogy is not perfect. One could argue that a better fit for the verbs in our "matter is like nouns" analogy is force. We'll talk about force another day.
[3] That's why it's called a motor—it generates motion—in the same way as a device that generates stamp collections is called a stamp collector.
[4] You probably know this is related to something called friction. The details of friction are, again, a story for another day. Sorry I keep doing this, but when you're setting up the fundamentals of a long story, some things have to wait. Ay, there's the rub...
|
LEGO® is a registered trademark of the LEGO Group of companies,
which does not sponsor, authorise, or endorse this site. This material is presented in accordance with the LEGO® Fair Play Guidelines. |