

| Archive Blog Cast Forum RSS Books! Poll Results About Search Fan Art Podcast More Stuff Random |
|
Classic comic reruns every day
|
1 {photo of a Sydney Opera House shells and the steps leading up to them}
1 Caption: Shells and energy levels
|
First (1) | Previous (3258) | Next (3260) || Latest Rerun (2891) |
Latest New (5380) First 5 | Previous 5 | Next 5 | Latest 5 Annotations theme: First | Previous | Next | Latest || First 5 | Previous 5 | Next 5 | Latest 5 This strip's permanent URL: http://www.irregularwebcomic.net/3259.html
Annotations off: turn on
Annotations on: turn off
|
 Rocks with gravitational potential energy. |
I've mentioned electron energy levels in atoms briefly, when discussing the mystery of the hydrogen spectrum and how quantum mechanics led to an explanation. A bit more thought reveals a wealth of phenomena that govern many of the dealings we have with light, as well as x-rays, radio, and other electromagnetic radiation.
The electrons surrounding an atom have a certain amount of potential energy. This is electrical potential energy, in the same way that objects held above the Earth's surface have gravitational potential energy. Gravity, if given the chance, can work on the object to make it fall. Similarly, the electrical attraction between the positively charged nucleus of the atom and the negatively charged electron can make the electron fall into the nucleus. Or it could if quantum mechanics didn't get in the way. Just as the surface of the Earth prevents objects falling all the way into the Earth's core, the laws of quantum mechanics provide a "surface" beyond which an electron cannot normally fall further into the nucleus.
Now think about the amounts of energy involved. For gravity, the potential energy is a minimum when the object is lying on the ground (assuming for now it can't possibly go any lower, so excluding the possibility of digging a hole, say). As the object is raised into the air, its gravitational potential energy increases. Keep going up, and the potential energy keeps going up. You can keep going up away from the Earth forever, but the total potential energy doesn't climb to infinity, because the gravitational strength of the Earth falls off faster than the height of the object. (In mathematical terms, gravity falls off as one over the distance squared.) The total potential energy of an object as far from the Earth as possible, and ignoring any effects from all the stars and galaxies and things that would actually dominate at that point, is a fixed, finite number.
 Adding gravitational potential energy to some weights. Creative Commons Attribution image by the U.S. Navy. |
So, after this digression, I can mention that the potential energy of an electron in the lowest energy quantum state around a hydrogen atom nucleus (i.e. a single proton) is conventionally said to be -13.6 eV. That is, if you pulled it right away from the proton, it would gain 13.6 eV of electrical potential energy. The eV stands for "electron volts", which is a unit of energy sized appropriately to measure the typical potential energies of electrons. One electron volt is the amount of electrical potential energy an electron would gain if you moved it from a region of positive charge to a region of negative charge, where the charge difference has a voltage of one volt.[1] An eV is about 1.6×10-19 joules of energy - a really tiny amount of energy compared to the sorts of things we humans deal with regularly.
 Shelves with differing amounts of potential energy. |
For a simple hydrogen atom, with just one electron, the electron can occupy any of the quantum shelves, or states. It prefers to sit in the ground state, for the same reason that objects prefer to sit on the ground rather than suspended in mid-air. Gravity pulls objects down, and electrical force pulls the electron in towards the ground state. Quantum states in the atom with higher energy than the ground state are called excited states, and we say that an electron in one of these states is excited. This is really saying that it has more energy than in the ground state - it's jumping around and generally having a party time, with that excess energy.
How can an electron get into an excited state? Quite simply, if it somehow gets enough extra energy to jump up from the ground state into one of the excited states. It can get this energy in a few different ways. One way is if the atoms are heated up. We've seen that heat is really the kinetic energy of the atoms in a material moving around. If you add enough heat, then the atoms will start slamming into one another more and more violently. At some point, these atomic collisions will have so much energy that an electron in one of the colliding atoms can absorb enough of it to jump up from the ground state into an excited state. In fact, if you heat things up a bit more, you can supply enough energy to bounce electrons completely off the atoms during these collisions. In other words, for our hydrogen example, you can give an electron 13.6 eV of energy or more, and the electron simply can't stay attached to its proton any more. If you strip electrons off atoms like this, we say you have ionised the atoms - turned them into ions. The resulting mix of hydrogen ions and loose electrons is known as a plasma, rather than a gas, and has very different properties because it can now interact strongly with electromagnetic fields.
 Remains of fallen rocks. |
E = hν = hc/λ,
where h is Planck's constant.
As it turns out, the energy difference between the ground state and every other state in a hydrogen atom is so large that any electron falling into the ground state produces a photon with a higher energy (and thus a higher frequency, or shorter wavelength) than visible light. The resulting photons are in fact ultraviolet light. However, an electron doesn't have to fall all the way in one go. Like tumbling down a set of stairs, it can fall from a high energy excited state into a lower energy excited state, pausing there for a while, before falling further. It turns out that the energy differences between the first excited state and higher excited states in hydrogen are the right size to give energies that correspond to visible light. It was these visible photons that Johannes Rydberg investigated in the 1880s. The energies (and hence wavelengths) of the photons emitted by electrons dropping down quantum states inside an atom are fixed by the energy levels of those states. So excited hydrogen atoms emit exactly four precise, distinct wavelengths of visible light, and no other. The other wavelengths that hydrogen atoms emit are in the ultraviolet or infrared.
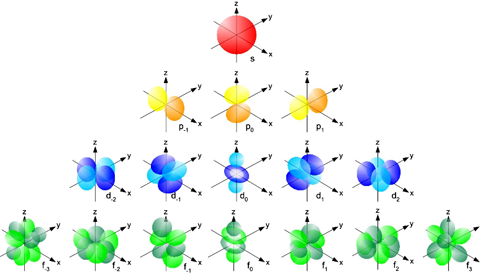 The shapes of the electron orbitals which have different energy levels. These are roughly the shapes of the fuzzy clouds for an individual electron around an atomic nucleus. Creative Commons Attribution-ShareAlike image by UC Davis ChemWiki. |
The second complication is that the energy levels of the quantum states vary for different atoms. More protons in the nucleus mean the lower energy states are more strongly held to the atom, resulting in lower energies than in hydrogen. On the other hand, the outermost electrons in heavier atoms are sitting on higher energy shelves (because the lower ones are full), so can be moved up or even stripped away completely with less energy. The result of all this is that the spectra of light emitted by different atoms are all very different from one another. In fact, just by looking at the spectrum of what light wavelengths are emitted by excited atoms of a particular element, you can identify the element with 100% reliability. One complication is that elements are rarely seen alone in pure form—they are mixed up with other elements—and you need to disentangle all the different wavelengths of light. There are other complications too, in that light can be generated by other means, such as black body radiation, and that the wavelengths of light can be shifted around by things such as the Döppler effect and scattering off tiny particles.
Nevertheless, identifying elements from their spectra (a technique called spectroscopy) is one of the most important scientific analysis tools we have today. Spectroscopy allows us to know what stars are made of and, on a grander scale, how they work. It is used to analyse biological and chemical samples, to determine causes of disease, to provide forensic evidence, and to perform fundamental medical research. It is used in engineering to analyse structures, to locate mineral resources, and to make sure our technological world doesn't fall down around us. It is used in geosciences to probe rocks, to study our atmosphere and oceans, and to understand the ecological impacts of natural and human-made events.
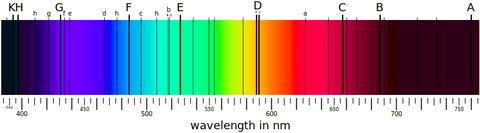 Spectrum of our sun in visible light. The dark lines correspond to various elements. A and B are oxygen; C, F, and G are three of the four visible hydrogen lines mentioned in the main text; D is a pair of sodium lines; E is iron; H and K are calcium. See Fraunhofer lines for the remainder. Public domain image from Wikimedia Commons. |
Black body radiation emits across all wavelengths. So if you have a black body—like a star for example—it produces a continuous spectrum of colours in the visible and extends into the infrared and ultraviolet too. If this light passes through a region of hydrogen gas, say, then the photons that happen to have the correct energies to excite some of the electrons get absorbed, while all of the other photons simply pass straight through the hydrogen unaffected. The result is that you have what looks like a continuous black body spectrum, with some dark lines at the wavelengths where photons have been removed. Those wavelengths are the same as the wavelengths that excited hydrogen atoms emit, so again you can uniquely identify the presence of hydrogen in this absorption spectrum.
Much of the work done in spectroscopy in fact looks at these sorts of absorption spectra, rather than emission spectra. The spectra of stars (including our own sun) are of this sort. You see a broad wash of black body radiation across all wavelengths, interrupted by numerous narrow black lines where various elements have absorbed the photons. We can match all of these lines up to determine what elements are in a star's atmosphere, and in our own atmosphere too.
 Layers of shells of different energy levels. |
Many of the phenomena we see around us every day come to our senses via photons. And many of those photons are constantly playing this game of absorption and emission with atoms. A lot of our technology works by making use of the interactions of electromagnetic waves with atoms. It's just another example of conversion of energy: from light into electrical potential energy, and back again. But it's at least as important as all the other things energy can get up to.
Oh, one more thing. If I've put it into your head that atoms have these things where electrons sit, and they're called shelves, as in an "electron shelf" - don't worry. The real name of these things is only a one letter difference: electron shell.
|
LEGO® is a registered trademark of the LEGO Group of companies,
which does not sponsor, authorise, or endorse this site. This material is presented in accordance with the LEGO® Fair Play Guidelines. |